Русский | English
Gallbladder bile formation |
|
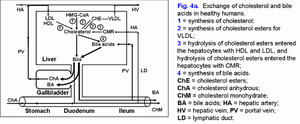
Share this: Read this article in PDF format Mechanism of gallbladder bile formationTwo points in the process of gallbladder bile formation should be distinguished: 1) in an fasting stomach; 2) after postprandial gallbladder emptying (1). The absorbing and concentrating functions determine the mechanism of the gallbladder bile formation. The rate of biliary cholesterol absorption by the gallbladder mucosa depends on the concentration of the cholesterol in the gallbladder bile (r= +0.60, p<0.001) (2-4). Taking into account the fact that the mixed (bile acids-phospholipid-cholesterol) micelles are not absorbed by the gallbladder mucosa, cholesterol can be absorbed as monomers or with phospholipid vesicles (1-9). The solubility of anhydrous cholesterol monomers in water is 0.013 nmol/ml, in the intermicellar phase – 0.260 nmol/ml, while in phospholipid vesicles - 5.5 μmol/ml (10-15). Therefore, according to the solubility of anhydrous cholesterol, it will be absorbed with the phospholipid vesicles to a greater degree (99.9%). The phospholipid vesicles can be absorbed by the gallbladder mucosa in different ways (1, 8, 9, 16-19). Therefore, the greater is the absorption of vesicular cholesterol by the gallbladder mucosa, the lower is the concentration of the cholesterol in the phospholipid vesicles of the gallbladder bile. The concentration function of the gallbladder consists in the accumulation of the bile acids of the hepatic bile in the gallbladder; it depends on the rate of bile acids of the hepatic bile entering the gallbladder and the rate of water absorption by the gallbladder mucosa, and it also determines the concentration of the total bile acids and the formation of mixed biliary micelles in the gallbladder bile (fig. 4a). In hepatic bile 40-80% of biliary cholesterol is in phospholipid vesicles and 20-60% of it is in mixed biliary micelles (15, 20, 21). The gallbladder, concentrating the bile acids, forms mixed biliary micelles and raises the level of biliary cholesterol in them up to 80-100% (15, 20, 21). Therefore, the greater is the absorption of water by the gallbladder mucosa, the grater is the passage of bile acids of hepatic bile to the gallbladder and the higher is the concentration of total bile acids in the gallbladder bile. Thus, the high concentration of the total bile acids and the low concentration of cholesterol in phospholipid vesicles result in the low cholesterol saturation index in the gallbladder bile (less than 1.0), which determines the stability of micellar carriers of biliary cholesterol and prevents the cholesterol monohydrate crystals from precipitating.
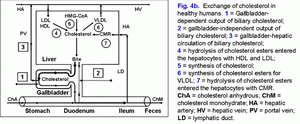
Fate of the absorbed vesicular biliary cholesterolPart of the absorbed cholesterol may be esterified in the epithelial cells of gallbladder mucosa by means of ACAT. Normally the activity of ACAT makes up 92±23 pmol/min per mg protein and is 8-9 times as high as that of microsomes of the liver (11±2 pmol/min per mg protein) (22). There is a positive correlation between the concentration of the cholesterol in the gallbladder bile and ACAT in microsomes of gallbladder mucosa (r= +0.42, p<0.05). The cholesterol is also synthesized in the microsomes of gallbladder mucosa, and the activity of HMG-CoA reductase in them makes up 28±6 pmol/min per mg protein. But it is 4 times lower, than that in the microsomes of hepatocytes (120±40 pmol/min per mg protein). The concentration of free cholesterol in the microsomes of gallbladder mucosa (206±9 nmol/min per mg protein) is 4 times higher than that in microsomes of hepatocytes (55±3 nmol/min per mg protein), while the concentration of esterified cholesterol (34±5 nmol/min per mg protein) is 3.5 times higher (9±1 nmol/min per mg protein) (22). Taking into account the low activity of HMG-CoA reductase and the high activity of ACAT in the microsomes of gallbladder mucosa, as well as the positive correlation between the level of cholesterol in the gallbladder bile and the microsomes of gallbladder mucosa (r= +0.75, p<0.01), the higher concentration of free cholesterol in the microsomes of epithelial cells of gallbladder mucosa may result only from the excessive absorption of the biliary vesicular cholesterol. It testifies that the rate of the biliary vesicular cholesterol entering the epithelial cells of gallbladder mucosa 4 times exceeds that of hepatocytes. By analogy with ileum, the removal of the absorbed vesicular cholesterol and phospholipids from the gallbladder wall may be realized by means of HDL and/or VLDL (17, 18, 23-25). It was shown in vitro that HDL are able to extract the excess of cholesterol out of cholesterol-saturated phospholipid vesicles and to dissolve the cholesterol crystals (26, 27). It is possibly that the mechanism of the removal of the absorbed vesicular cholesterol and phospholipids by means of HDL may be prevalent and it may be determined by the concentration of HDL in serum and by the rate of the arterial bloodstream in the gallbladder wall (28, 29). In the gallbladder wall HDL, interrelating with phospholipid vesicles, will extract biliary vesicular cholesterol and phospholipids and, with bloodstream, will first enter the gallbladder vein and then, through the portal vein, the liver (fig. 4b). This supposition is indirectly corroborated by the negative correlative connection between CSI and the level of the total cholesterol (TCh) (r= .0.65, p<0.05) and Ch-HDL (r= .0.62, p<0.05) in the serum in practically healthy women (30). In the gallbladder mucosa, small quantities of VLDL synthesize (31). The number of apoproteins B, C-II and C-III absorbed by the gallbladder mucosa can make up 84-91% of total quantity of apoproteins B, C-II and C-III, entering the gallbladder with the hepatic bile (32). Also, taking into account that the mucosa of the gallbladder absorbs phospholipid vesicles, apoproteins B, C-II and C-III, they can interrelate with serum HDL, LDL, and VLDL in the gallbladder wall below epithelium. The absorbed vesicular cholesterol of gallbladder mucosa, interrelating with blood lipoproteins, can enter the liver or the peripheral blood stream through the portal vein (fig. 4.a). The way of biliary cholesterol [blood (lipoproteins) → liver (hepatic bile - phospholipid vesicles) → gallbladder (absorption of vesicular cholesterol) → portal vein (lipoproteins) → liver or blood] - was called by us “gallbladder-hepatic circulation of biliary cholesterol” (fig. 4.a). The detailed structuring of these processes provides an opportunity to connect the excretory function of the liver and the absorption and evacuation functions of the gallbladder with the level of cholesterol in serum.
References:
|
|
|