Русский | English
Physiology of bile |
|
Share this: Read this article in PDF format Functions of the gallbladderThe prevalent point of view is that gallbladder is not essential for life (1). The gallbladder has the absorption, concentration, secretion, and evacuation functions (2, 3). The absorption and concentration functions are interdependent. The absorption function of the gallbladder includes the absorption of water, Na+, cholesterol, phospholipids, hydrophilic proteins, etc (4-14). Since the absorption of the bile acids by the gallbladder mucosa is 2-6% of the total concentration in the gallbladder bile, the concentration function of the gallbladder consists in the accumulation of bile acids of hepatic bile in the gallbladder (10-12, 15, 16). The secretion function of the gallbladder includes the secretion of glycoprotein mucin by the gallbladder mucosa, Н+ ions, Cl- and probably immunoglobulins and Ca2+ (5, 17-23).
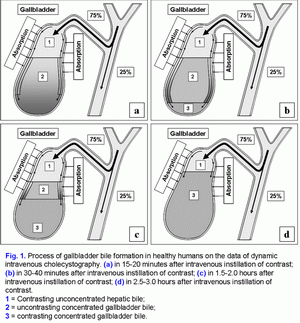
Conceptual model of gallbladder bile formationConsidering the fact that the detailed structuring of the process of hepatic bile entering the gallbladder has not been worked out, we have introduced two new terms into practice: the “active” and “passive” passages of the hepatic bile. The “active” passage depends on the ejection volume of the gallbladder after meal or during the interdigestive period. The “passive” passage is connected with the rate of water absorption in the gallbladder. Hence the rate of the hepatic bile entering the gallbladder contains both the “active” and the “passive” passages. During the “active” passage only one volume (out of 6-9) of the hepatic bile enters versus 5-8 volumes during the “passive” passage. The rate of hepatic bile entering the gallbladder depends on the absorption rate of water by the gallbladder mucosa (r=+0.99, р<0.001) (24). The absorption rate of water by the gallbladder mucosa is up to 100-250 μl/min; sometimes it may increases up to 500 μl/min (4). The rate of hepatic bile entering the gallbladder is 75% of the basal secretion of hepatic bile (24). It is indirectly confirmed by the fact that 78±10% of bile acids from their total pool is accumulated in the gallbladder (25). The concentration of total bile acids in the gallbladder bile depends on the rate of bile acids of hepatic bile entering the gallbladder (r=+0.87, р<0.001) (24). Detailed structuring of the process of hepatic bile entering the gallbladder suggests that 83-89% of the bile acids, contained in gallbladder bile, enters during the “passive” passage, and only 11-17% of bile acids during the “active” passage. Hence, the “passive” passage of hepatic bile into the gallbladder plays an important role in the mechanism of gallbladder bile formation (fig. 1.a). Normally the process of the gallbladder filling after the intravenous introduction of X-ray contrast is characterized by some regular features (26). During the first 15-20 minutes the gallbladder bile has two layers: the upper contrasting and the low uncontrasting (fig. 1.a). The legible border between them is situated horizontally. During the 30-40th minute the upper layer contrasting bile near the wall thickens, its density grows because of the presence of iodine heavy atoms and exceeds the density of the uncontrasting concentrated bile. Besides, the “heavy” layers of the contrasting bile begins to trickle down along the walls, as if flowing round the uncontrasting concentrated bile, and accumulate at the fundus (fig. 1.b). The gallbladder shadow becomes three-layered: the contrasting, but unconcentrated bile above, the concentrated, but uncontrasting bile underneath and the contrasting and concentrated bile after the lower part of gallbladder. The boundary between them is legible and it does not change if a patient moves. The quantity of the concentrated contrasting bile at the fundus of the gallbladder increases gradually, and the upper boundary of the lower layer rises (fig. 1.c). The gallbladder shadow gains homogeneity 2.5-3.0 hours after the moment of the contrast introduction (fig. 1.d) (26). Therefore, in a fasting state at night period or an interdigestive state the absorption of water by the infundibulum mucosa of the gallbladder plays the leading role in gallbladder bile formation (unpublished data).
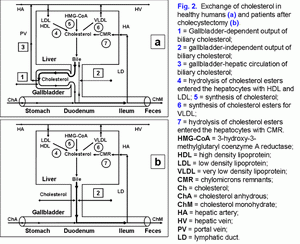
Outflow of biliary cholesterol into duodenumFor understanding the processes of the biliary cholesterol outflow into the duodenum, we have introduced two new terms, namely: the gallbladder-dependent and gallbladder-independent output of biliary cholesterol. The former depends on the ejection volume of the gallbladder and the concentration of biliary cholesterol in the gallbladder bile; the latter depends on the concentration of biliary cholesterol in the hepatic bile entering directly the duodenum (fig. 2.a). After cholecystectomy only gallbladder-independent output of biliary cholesterol to the duodenum is observed (fig. 2.b).
Interdependence between the absorption of biliary cholesterol in the gallbladder and that of the ileumIn the gallbladder, vesicular cholesterol absorbs effectively, but micellar cholesterol does not (7-12, 24, 27, 28). The absorption of micelles in the ileum is 100 times more effective than that of vesicles (29). Hence, the greater is the absorption of vesicular cholesterol in the gallbladder, the higher is the concentration of micellar cholesterol in the gallbladder bile (CSI < 1.0) and the absorption of cholesterol in the ileum. Vice versa, the decrease of the vesicular cholesterol absorption in the gallbladder raises the vesicular cholesterol concentration in the gallbladder bile (CSI more than 1.0) and reduces the cholesterol absorption in the ileum.The ratio bile acids/cholesterol in the gallbladder bile may determine the ability of intestinal mixed micelles to solubilize dietary cholesterol. The rise of this ratio by more than 10-12: 1 (CSI < 1.0) results in the increase of the solubilization, and its decrease by less than 7-10: 1 (CSI > 1.0) results in the reduction of the solubilization.
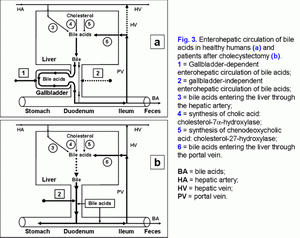
Effect of gallbladder functions on enterohepatic circulationPart of the bile acids of the hepatic bile enters the gallbladder and is accumulated in it; the other part enters the duodenum and participates in the enterohepatic circulation.To understand these processes, we have introduced two new terms: gallbladder-dependent and gallbladder-independent enterohepatic circulation of bile acids (fig. 3.a). The gallbladder-dependent enterohepatic circulation of bile acids depends on the ejection volume of the gallbladder and determines the concentration of bile acids of the gallbladder bile that participate in the enterohepatic circulation. The gallbladder-independent enterohepatic circulation includes the part of bile acids of the hepatic bile that enter directly the duodenum, but not the gallbladder. In healthy people 75-80% of bile acids participate in the gallbladder-dependent enterohepatic circulation, and only 20-25% of bile acids take part in the gallbladder-independent circulation (fig. 3.a). Therefore, the concentration function of the gallbladder consists in the accumulation of bile acids of the hepatic bile and their exclusion from the enterohepatic circulation. The part of bile acids participating in the gallbladder-independent enterohepatic circulation after cholecystectomy increases up to 100% (fig. 3.b). Detailed structuring of these processes enables to connect the absorption, concentration and evacuation functions of the gallbladder with the enterohepatic circulation of bile acids. The rate of water absorption by the gallbladder mucosa determines the passive passage of the hepatic bile from the liver into the gallbladder and the gallbladder-independent enterohepatic circulation of the bile acids.
References:
|
|
|