|
Share this:
Read this article in PDF format
Chronic acalculous cholecystitis with biliary sludge is an inflammatory disease, which affects the gallbladder wall and causes motoric-tonic dysfunction of the biliary tract, which is accompanied by appearance of hyperechogenic particles in the gallbladder lumen and reveals as biliary pain (1, 2). The cause of the gallbladder motility disorders can be caused by increased basal cystic duct resistance or cystic duct spasm, muscle hypertrophy, and chronic aseptic inflammation in the gallbladder wall.
“Biliary sludge” is designated as any heterogeneity of gallbladder bile found with ultrasound examination (2). Biliary sludge is the result of precipitation of calcium bilirubinate granules, cholesterol monohydrate crystals and polymerized glycoprotein biliary mucin in gallbladder bile (3-7). Only cholesterol monohydrate crystals are the hyperehogenic particles without an acoustic shadow at sonography (3, 5).
Biliary sludge is often found in women during pregnancy, in obese patients on a very-low-calorie diet (rapid weight loss), in patients with high spinal cord injuries, prolonged total parenteral nutrition, prolonged treatment with octreotide, and in patients after gastrectomy or colectomy (8).
According to the position in the gallbladder and the form biliary sludge is classified in diffused, surface, and precipitating (9). The dynamics of the transformation of biliary sludge into cholesterol gallstones has been shown as follows: diffused biliary sludge → precipitating biliary sludge → a cholesterol gallstone without acoustic shadow → a cholesterol gallstone with acoustic shadow (9). The transformation time of biliary sludge into cholesterol gallstones in the gallbladder makes up 3-36 months (8). Depending on cause the percentage of transformation may change from 5% to 50%.
Biliary sludge and cholesterol gallstones are formed in 25-50% of obese patients during very-low-calorie diet within 3-6 months, in 40% of obese patients within 6 months after surgery (8). Approximately 25% of obese patients who undergo strict dietary restriction develop gallstones, and as many as 50% of patients who undergo gastric bypass develop gallbladder sludge or gallstones within six months after surgery (8). As many as 40% of these patients will develop symptoms related to gallstones within the some six-month period (8). A decreased incidence in gallstone formation of 28% to 3% in obese patients on a very-low-calorie diet when they received 600 mg/day ursodeoxycholic acid (8). As many as 45% of adults will develop gallstones after 3 to 4 months of total parenteral nutrition (8).
The incidence of new sludge and stone formation during pregnancy is approximately 30% and 2%, respectively (8). After delivery, gallbladder motility returns to normal and biliary sludge disappears in 60% to 70% and stone disappears in 20% to 30% of women (8). In patients with biliary sludge who were followed prospectively, 8% were shown to develop asymptomatic gallstones and 6% developed symptomatic stones requiring cholecystectomy after 38 months (8). In 18% of the patients, the biliary sludge disappeared spontaneously; in the remaining 60%, the biliary sludge disappeared and subsequently reappeared (8).
Complications such as acute cholecystitis have been reported to occur in as many as 20% of patients with biliary sludge (8). It is clear from these and other studies that biliary sludge can be a precursor to stone formation and can be a source of potential complications (8).
Russian gastroenterologists single out 3 main types of biliary sludge, which have most clear ultrasound descriptions (2):
- Microlithiasis is a presence of movable hyperechogenic single or multiple dot particles without acoustic shadow in the gallbladder lumen.
- Echo unhomogeneous gallbladder bile includes movable clots of different densities without acoustic shadow in the gallbladder lumen.
- Combination of “putty-like” bile with microlits. These microlits can be present simultaneously as in the “putty-like” bile, and as well as in the gallbladder lumen.
Diagnostic criteria of the chronic acalculous cholecystitis with biliary sludge
- Biliary pains and feeling of discomfort in the right hypochondrium. Biliary pains may be in the right hypochondrium, frequently or occasionally, of different intensity and duration, no related or related to intake of fatty meals (1).
In addition, biliary pain may occur with one or more of the following symptoms:
- regular or periodical feeling of bitter taste in the mouth
- nausea, heartburn, eructation, vomiting
- regular or periodical abdominal distension and borborygmus
- unstable stool with alternation of constipation or diarrhea
- pain occurs at night or after a meal
- Impaired gallbladder emptying.
- According to ultrasound examination, thickening of the gallbladder wall up to 3-6 mm.
Causes of the gallbladder evacuation dysfunction, biliary pain and chronic aseptic inflammation in the gallbladder wall
- Pathology of the smooth muscle cells and epithelial cells in the gallbladder wall (high degree of COX-2 expression in the smooth muscle cells and epithelial cells of the gallbladder wall).
- Hypersecretion of glycoprotein biliary mucin into gallbladder lumen and increase in concentration of glycoprotein biliary mucin in the gallbladder bile over point of polymerization (>2.0 mg/ml) (high degree of COX-2 expression in the epithelial cells of the gallbladder mucosa).
- Contractile dyscoordination of the gallbladder and cystic duct (surplus degree of COX-2 expression in the smooth muscle cells of the gallbladder and cystic duct).
- Increased basal cystic duct resistance (high degree of COX-2 expression in the smooth muscle cells of the cystic duct).
- Increased basal common bile duct resistance (high degree of COX-2 expression in the smooth muscle cells of the sphincter of Oddi).
Mechanism of development of pathologic disorders
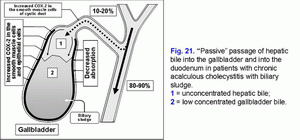
High degree of COX-2 expression in the smooth muscle cells of the gallbladder wall causes decrease in the evacuation function of the gallbladder and “active” passage of the hepatic bile into the gallbladder (fig. 21).
Surplus COX-2 expression in the epithelial cells of the gallbladder mucosa causes decrease in the absorption function of the gallbladder (decrease of water and biliary cholesterol absorption) and “passive” passage of the hepatic bile into the gallbladder (fig. 21).
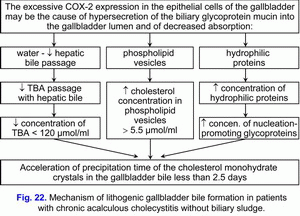
This is accompanied by decrease in concentration of total bile acids in the gallbladder bile and increase in concentration of biliary cholesterol in the phospholipid vesicles, and causes disturbance in colloidal stability of the gallbladder bile and precipitation of cholesterol monohydrate crystals from unstable multilamellar aggregated phospholipid vesicles and calcium bilirubinate granules, i.e. formation of “lithogenic” gallbladder bile (fig. 22).
High degree of COX-2 expression in the epithelial cells of the gallbladder mucosa causes hypersecretion of glycoprotein mucin into the gallbladder lumen and gallbladder bile. Increase in concentration of glycoprotein biliary mucin in the gallbladder bile over 2.0 mg/ml helps in its polymerization and formation of sites of the excessive viscosity and it is accompanied by rise of gallbladder bile viscosity. Precipitation of cholesterol monohydrate crystals and calcium bilirubinate granules in the sites of the excessive viscosity of polymerized glycoprotein biliary mucin causes formation of biliary sludge, increase its echogenicity and its reveal during ultrasonographic examination.
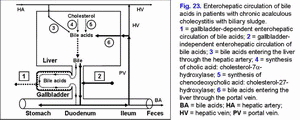
Decrease in “active” and “passive” passage of the hepatic bile into the gallbladder causes increase in passage of hepatic bile into the duodenum and gallbladder-independent enterohepatic circulation of bile acids, biliary cholesterol and biliary bilirubin (fig. 23).
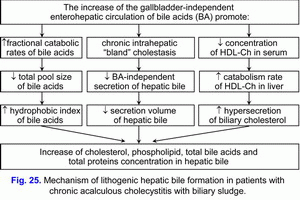
Increase in the gallbladder-independent enterohepatic circulation of bile acids causes increase in concentration of bile acids in the hepatocytes and decrease in the accumulation function and excretion function of liver (i.e. formation of chronic “bland” intrahepatic cholestasis) (fig. 23).
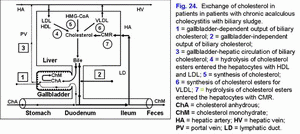
Increase in the gallbladder-independent enterohepatic circulation of biliary cholesterol helps in increase in absorption of biliary cholesterol in the small intestine, the biliary cholesterol entering hepatocytes, and the hypersecretion biliary cholesterol into hepatic bile (fig. 24).
These two factors contribute to the formation of the “lithogenic” hepatic bile (fig. 25).
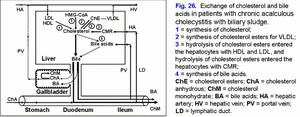
Decrease in the gallbladder-dependent output of biliary cholesterol and in the concentration of total bile acids in the gallbladder bile results in formation of the “lithogenic” gallbladder bile and precipitation of the cholesterol monohydrate crystals in the gallbladder lumen in 100% of patients with chronic acalculous cholecystitis with biliary sludge (fig. 26).
Long-term storage of biliary sludge and gallbladder hypomotility contributes to the gallstones formation in the gallbladder and transformation of chronic acalculous cholecystitis with biliary sludge into chronic calculous cholecystitis.
Pathogenetic treatment of patients with chronic acalculous cholecystitis with biliary sludge
Accordingly, treatment of the chronic acalculous cholecystitis with biliary sludge (with biliary pain) aiming for the prophylactics of the chronic calculous cholecystitis, duodeno-gastral reflux, antral atrophic (bile acid-dependent) gastritis and chronic biliary pancreatitis includes:
- Celecoxib - 100 mg, 2 times a day after meal for 5-7 days, after which
- Ursodeoxycholic acid - 750 mg, once a day (in the evening) for 2 month.
Celecoxib is a selective inhibitor of COX-2. Inhibiting COX-2 activity in the smooth muscle cells of the gallbladder wall and cystic duct it brings relief of the biliary pain within 3-5 days, restoration of the evacuation function of the gallbladder and the gallbladder-dependent output of the biliary cholesterol, “active” and “passive” passage of the hepatic bile into the gallbladder, and decrease in the gallbladder-independent enterohepatic circulation of bile acids, biliary cholesterol and biliary bilirubin.
Celecoxib, a selective inhibitor of COX-2, inhibiting COX-2 activity in the epithelial cells of the gallbladder mucosa causes inhibition in glycoprotein mucin hypersecretion into the gallbladder lumen, decrease in concentration of glycoprotein biliary mucin in gallbladder bile and gallbladder bile viscosity, which prevents formation of biliary sludge.
Low COX-2 activity in the epithelial cells of the gallbladder mucosa helps in restoration of the absorption function of the gallbladder (absorption of water and biliary cholesterol), which results in increase in concentration of total bile acids and decrease in concentration of biliary cholesterol in gallbladder bile.
Ursodeoxycholic acid, is a hydrophilic hepatoprotective bile acid. It helps in dissolving the cholesterol monohydrate crystals in the gallbladder, decrease in lithogenicity of gallbladder and hepatic bile, disappearance of the chronic “bland” intrahepatic cholestasis (i.e. results in restoration of the accumulation and excretion functions of liver).
Celecoxib and Ursodeoxycholic acid, blocking main pathogenetic mechanisms of gallstones formation, contribute to elimination of biliary sludge in 100% of cases, and lower the repeated risk of biliary sludge formation, and, respectively, reduce risk of gallstone formation in the gallbladder.
Celecoxib is a selective inhibitor of COX-2. Inhibiting COX-2 activity in the smooth muscle cells of the biliary tract and the sphincter of Oddi it brings relief of the biliary pain within 3-5 days, restoration of the passage of the hepatic bile into the duodenum.
Celecoxib is a selective inhibitor of COX-2, inhibiting COX-2 activity in the epithelial cells of the biliary tract mucosa causes decrease in secretion of glycoprotein mucin into the biliary tract lumen, concentration of the glycoprotein biliary mucin in the hepatic bile and viscosity of hepatic bile, which prevents formation of biliary sludge and gallstones in the common hepatic duct and common bile duct. Low COX-2 activity in the epithelial cells and the smooth muscle cells of the biliary tract helps in lowering the risk of choledocholithiasis development.
Ursodeoxycholic acid (UDCA) is a hydrophilic hepatoprotective bile acid. It helps in dissolving the cholesterol monohydrate crystals in the biliary tract, decrease in lithogenicity of hepatic bile, disappearance of the chronic “bland” intrahepatic cholestasis (i.e. results in the restoration of the accumulation and excretion functions of liver), and in some patients helps in dissolving the biliary sludge in the biliary tract.
Ursodeoxycholic acid (UDCA) is a hydrophilic hepatoprotective bile acid, decreasing aggressive properties of bile, prevents development of chronic atrophic antral gastritis (duodenogastric reflux and bile reflux gastritis) and duodeno-gastroesophageal reflux (incompetence of Oddi's sphincter), chronic biliary pancreatitis (biliopancreatic reflux) or chronic spastic aseptic pancreatitis (pancreatic type III of sphincter of Oddi dysfunction).
Celecoxib and Ursodeoxycholic acid (UDCA), pathogenetically blocking main mechanisms of gallstone formation, help in prophylactics of gallstone formation in the biliary tract, and lower the risk of development of choledocholithiasis and chronic biliary pancreatitis (1-66).
Effectiveness is 95%.
Remission period is 18-24 months.
Attention!!! Information for patients:
Before using this scheme of treatment please check the contraindications (below) and side effects of using pharmacological preparations of Celecoxib and Ursodeoxycholic acid, and obtain your doctor’s permission.
Contraindications for Celecoxib:
- allergic reactions (nettle-rash, bronchial spasm) to acetylsalicylic acid or other NSAIDs (in anamnesis);
- 3rd trimester of pregnancy;
- high sensitivity to sulphonamides;
- high sensitivity to any component of the preparation.
Contraindications for Ursodeoxycholic acid:
- high sensitivity to the preparation;
- acute inflammatory diseases of the gallbladder and the bile ducts;
- ulcerative colitis;
- Crone’s disease.
This web page does not bear any legal responsibility for the use of the proposed treatment schemes without consulting your doctor.
References:
- General practitioner: gastroenterology. 2002; 1: 22.
- Ilchenko A.A., Delyukina O.V. Clinical role of biliary sludge. Consilium Medicum. Gastroenterology. 2005; 2.
- Lee S.P., Nicholls J.F. Nature and composition of biliary sludge. Gastroenterology. 1986; 90: 677-686.
- Carey M.C., Cahalane M.J. Whither biliary sludge? Gastroenterology. 1988; 95: 508-523.
- Wilkinson L.S., Levine T.S., Smith D., Chadwick S.J. Biliary sludge: can ultrasound reliably detect the presence of crystals in bile? Europ. J. Gastroenterol. Hepatol. 1996; 8: 999-1001.
- Jungst D., del Pozo R., Christoph S. et al. Quantification of biliary “sludge” in patients with cholesterol, mixed and pigment stones. Gastroenterology. 1994; 106(4): Abstr. 912.
- Jungst D., del Pozo R., Christoph S. et al. Sedimentation of biliary sludge. Effect on composition of gallbladder bile from patients with cholesterol, mixed and pigment stones. Scand. J. Gastroenterol. 1996; 31: 273-278.
- Bilhartz L.E., Horton J.D. Gallstone disease and its complications. In: M. Feldman, B.F. Scharschmidt, M.H. Sleisenger, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 6th ed. Philadelphia: WB Saunders Company, 1998: 948-972.
- Inoue K., Fuchigami A., Higashide S. et al. Gallbladder sludge and stone formation in relation to contractile function after gastrectomy. Ann. Surg. 1992; 215: 19-26.
References (Celecoxib and UDCA):
- Chen XW, Cai JT. The impact of selective cycloxygenase-2 inhibitor celexibo on the formation of cholesterol gallstone. Zhonghua Nei Ke Za Zhi. 2003; 42(11): 797-9.
- Guarino MP, Carotti S, Sarzano M, Alloni R, Vanni M, Grosso M, Sironi G, Maffettone PL, Cicala M. Short-term ursodeoxycholic acid treatment improves gallbladder bile turnover in gallstone patients: a randomized trial. Neurogastroenterol Motil. 2005; 17(5): 680-6.
- Pazzi P, Petroni ML, Prandini N, Adam JA, Gullini S, Northfield TC, Jazrawi RP. Postprandial refilling and turnover: specific gallbladder motor function defects in patients with gallstone recurrence. Eur J Gastroenterol Hepatol. 2000; 12(7): 787-94.
- Ikegami T, Matsuzaki Y, Fukushima S, Shoda J, Olivier JL, Bouscarel B, Tanaka N. Suppressive effect of ursodeoxycholic acid on type IIA phospholipase A2 expression in HepG2 cells. Hepatology. 2005; 41(4): 896-905.
- Shoda J, Ueda T, Kawamoto T, Todoroki T, Asano T, Sugimoto Y, Ichikawa A, Maruyama T, Nimura Y, Tanaka N. Prostaglandin E receptors in bile ducts of hepatolithiasis patients and the pathobiological significance for cholangitis. Clin Gastroenterol Hepatol. 2003; 1(4): 285-96.
- Shoda J, Kano M, Asano T, Irimura T, Ueda T, Iwasaki R, Furukawa M, Kamiya J, Nimura Y, Todoroki T, Matsuzaki Y, Tanaka N. Secretory low-molecular-weight phospholipases A2 and their specific receptor in bile ducts of patients with intrahepatic calculi: factors of chronic proliferative cholangitis. Hepatology. 1999; 29(4): 1026-36.
- Tomida S, Abei M, Yamaguchi T, Matsuzaki Y, Shoda J, Tanaka N, Osuga T. Long-term ursodeoxycholic acid therapy is associated with reduced risk of biliary pain and acute cholecystitis in patients with gallbladder stones: a cohort analysis. Hepatology. 1999; 30(1): 6-13.
- Kano M, Shoda J, Irimura T, Ueda T, Iwasaki R, Urasaki T, Kawauchi Y, Asano T, Matsuzaki Y, Tanaka N. Effects of long-term ursodeoxycholate administration on expression levels of secretory low-molecular-weight phospholipases A2 and mucin genes in gallbladders and biliary composition in patients with multiple cholesterol stones. Hepatology. 1998; 28(2): 302-13.
- Shoda J, Ueda T, Ikegami T, Matsuzaki Y, Satoh S, Kano M, Matsuura K, Tanaka N. Increased biliary group II phospholipase A2 and altered gallbladder bile in patients with multiple cholesterol stones. Gastroenterology. 1997; 112(6): 2036-47.
- Carotti S, Guarino MP, Cicala M, Perrone G, Alloni R, Segreto F, Rabitti C, Morini S. Effect of ursodeoxycholic acid on inflammatory infiltrate in gallbladder muscle of cholesterol gallstone patients. Neurogastroenterol Motil. 2010.
- Guarino MP, Carotti S, Morini S, Perrone G, Behar J, Altomare A, Alloni R, Caviglia R, Emerenziani S, Rabitti C, Cicala M. Decreased number of activated macrophages in gallbladder muscle layer of cholesterol gallstone patients following ursodeoxycholic acid. Gut. 2008; 57(12): 1740-1.
- Jüngst C, Sreejayan N, Zündt B, Müller I, Spelsberg FW, Hüttl TP, Kullak-Ublick GA, del Pozo R, Jüngst D, von Ritter C. Ursodeoxycholic acid reduces lipid peroxidation and mucin secretagogue activity in gallbladder bile of patients with cholesterol gallstones. Eur J Clin Invest. 2008; 38(9): 634-9.
- Spier BJ, Pfau PR, Lorenze KR, Knechtle SJ, Said A. Risk factors and outcomes in post-liver transplantation bile duct stones and casts: A case-control study. Liver Transpl. 2008; 14(10): 1461-5.
- Guarino MP, Cong P, Cicala M, Alloni R, Carotti S, Behar J. Ursodeoxycholic acid improves muscle contractility and inflammation in symptomatic gallbladders with cholesterol gallstones. Gut. 2007; 56(6): 815-20.
- Mas MR, Comert B, Mas N, Yamanel L, Ozotuk H, Tasci I, Jazrawi RP. Effects of long term hydrophilic bile acid therapy on in vitro contraction of gallbladder muscle strips in patients with cholesterol gallstones. World J Gastroenterol. 2007; 13(32): 4336-9.
- Jüngst C, Sreejayan N, Eder MI, von Stillfried N, Zündt B, Spelsberg FW, Kullak-Ublick GA, Jüngst D, von Ritter C. Lipid peroxidation and mucin secretagogue activity in bile of gallstone patients. Eur J Clin Invest. 2007; 37(9): 731-6.
- Itoh S, Kono M, Akimoto T. Psoriasis treated with ursodeoxycholic acid: three case reports. Clin Exp Dermatol. 2007; 32(4): 398-400.
- Beuers U. Drug insight: Mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2006; 3(6): 318-28.
- Colecchia A, Mazzella G, Sandri L, Azzaroli F, Magliuolo M, Simoni P, Bacchi-Reggiani ML, Roda E, Festi D. Ursodeoxycholic acid improves gastrointestinal motility defects in gallstone patients. World J Gastroenterol. 2006; 12(33): 5336-43.
- Pemberton PW, Aboutwerat A, Smith A, Warnes TW. Ursodeoxycholic acid in primary biliary cirrhosis improves glutathione status but fails to reduce lipid peroxidation. Redox Rep. 2006; 11(3): 117-23.
- Jeong HJ, Kim CG. Pretreatment with ursodeoxycholic acid (UDCA) as a novel pharmacological intervention in hepatobiliary scintigraphy. Yonsei Med J. 2005; 46(3): 394-8.
- Fischer S, Müller I, Zündt BZ, Jüngst C, Meyer G, Jüngst D. Ursodeoxycholic acid decreases viscosity and sedimentable fractions of gallbladder bile in patients with cholesterol gallstones. Eur J Gastroenterol Hepatol. 2004; 16(3): 305-11.
- Sauter GH, Thiessen K, Parhofer KG, Jüngst C, Fischer S, Jüngst D. Effects of ursodeoxycholic acid on synthesis of cholesterol and bile acids in healthy subjects. Digestion. 2004; 70(2): 79-83.
- Xiao ZL, Biancani P, Carey MC, Behar J. Hydrophilic but not hydrophobic bile acids prevent gallbladder muscle dysfunction in acute cholecystitis. Hepatology. 2003; 37(6): 1442-50.
- Tazuma S, Nishioka T, Ochi H, Hyogo H, Sunami Y, Nakai K, Tsuboi K, Asamoto Y, Sakomoto M, Numata Y, Kanno K, Yamaguchi A, Kobuke T, Komichi D, Nonaka Y, Chayama K. Impaired gallbladder mucosal function in aged gallstone patients suppresses gallstone recurrence after successful extracorporeal shockwave lithotripsy. J Gastroenterol Hepatol. 2003; 18(2): 157-61.
- Gunsar C, Melek M, Karaca I, Sencan A, Mir E, Ortac R, Canan O. The biochemical and histopathological effects of ursodeoxycholic acid and metronidazole on total parenteral nutrition-associated hepatic dysfunction: an experimental study. Hepatogastroenterology. 2002; 49(44): 497-500.
- Xiao ZL, Rho AK, Biancani P, Behar J. Effects of bile acids on the muscle functions of guinea pig gallbladder. Am J Physiol Gastrointest Liver Physiol. 2002; 283(1): G87-94.
- Kano M, Shoda J, Satoh S, Kobayashi M, Matsuzaki Y, Abei M, Tanaka N. Increased expression of gallbladder cholecystokinin: a receptor in prairie dogs fed a high-cholesterol diet and its dissociation with decreased contractility in response to cholecystokinin. J Lab Clin Med. 2002; 139(5): 285-94.
- Wang DQ, Tazuma S. Effect of beta-muricholic acid on the prevention and dissolution of cholesterol gallstones in C57L/J mice. J Lipid Res. 2002; 43(11): 1960-8.
- Lukivskaya OY, Maskevich AA, Buko VU. Effect of ursodeoxycholic acid on prostaglandin metabolism and microsomal membranes in alcoholic fatty liver. Alcohol. 2001; 25(2): 99-105.
- Bomzon A, Ljubuncic P. Ursodeoxycholic acid and in vitro vasoactivity of hydrophobic bile acids. Dig Dis Sci. 2001; 46(9): 2017-24.
- Sunami Y, Tazuma S, Kajiyama G. Gallbladder dysfunction enhances physical density but not biochemical metastability of biliary vesicles. Dig Dis Sci. 2000; 45(12): 2382-91.
- Ljubuncic P, Said O, Ehrlich Y, Meddings JB, Shaffer EA, Bomzon A. On the in vitro vasoactivity of bile acids. Br J Pharmacol. 2000; 131(3): 387-98.
- Nishioka T, Tazuma S, Yamashita G, Kajiyama G. Partial replacement of bile salts causes marked changes of cholesterol crystallization in supersaturated model bile systems. Biochem J. 1999; 340 ( Pt 2): 445-51.
- Sinisalo J, Vanhanen H, Pajunen P, Vapaatalo H, Nieminen MS. Ursodeoxycholic acid and endothelial-dependent, nitric oxide-independent vasodilatation of forearm resistance arteries in patients with coronary heart disease. Br J Clin Pharmacol. 1999; 47(6): 661-5.
- van de Heijning BJ, van de Meeberg PC, Portincasa P, Doornewaard H, Hoebers FJ, van Erpecum KJ, Vanberge-Henegouwen GP. Effects of ursodeoxycholic acid therapy on in vitro gallbladder contractility in patients with cholesterol gallstones. Dig Dis Sci. 1999; 44(1): 190-6.
- Mendez-Sanchez N, Brink MA, Paigen B, Carey MC. Ursodeoxycholic acid and cholesterol induce enterohepatic cycling of bilirubin in rodents. Gastroenterology. 1998; 115(3): 722-32.
- Benedetti A, Alvaro D, Bassotti C, Gigliozzi A, Ferretti G, La Rosa T, Di Sario A, Baiocchi L, Jezequel AM. Cytotoxicity of bile salts against biliary epithelium: a study in isolated bile ductule fragments and isolated perfused rat liver. Hepatology. 1997; 26(1): 9-21.
- Ohtake M, Sandoh N, Sakaguchi T, Tsukada K, Hatakeyama K. Enhancement of portal blood flow by ursodesoxycholic acid in partially hepatectomized rats. Surg Today. 1996; 26(2): 142-4.
- Fahey DA, Carey MC, Donovan JM. Bile acid/phosphatidylcholine interactions in mixed monomolecular layers: differences in condensation effects but not interfacial orientation between hydrophobic and hydrophilic bile acid species. Biochemistry. 1995; 34(34): 10886-97.
- Bouscarel B, Ceryak S, Robins SJ, Fromm H. Studies on the mechanism of the ursodeoxycholic acid-induced increase in hepatic low-density lipoprotein binding. Lipids. 1995; 30(7): 607-17.
- Bomzon A, Ljubuncic P. Bile acids as endogenous vasodilators? Biochem Pharmacol. 1995; 49(5): 581-9.
- Jazrawi RP, Pazzi P, Petroni ML, Prandini N, Paul C, Adam JA, Gullini S, Northfield TC. Postprandial gallbladder motor function: refilling and turnover of bile in health and in cholelithiasis. Gastroenterology. 1995; 109(2): 582-91.
- Pak JM, Adeagbo AS, Triggle CR, Shaffer EA, Lee SS. Mechanism of bile salt vasoactivity: dependence on calcium channels in vascular smooth muscle. Br J Pharmacol. 1994; 112(4): 1209-15.
- Sasaki H, Tazuma S, Kajiyama G. Effects of 16,16-dimethyl prostaglandin E2 on biliary mucous glycoprotein and gallstone formation in guinea pigs. Scand J Gastroenterol. 1993; 28(6): 495-9.
- Mizuno S, Tazuma S, Kajiyama G. Stabilization of biliary lipid particles by ursodeoxycholic acid. Prolonged nucleation time in human gallbladder bile. Dig Dis Sci. 1993; 38(4): 684-93.
- Pak JM, Lee SS. Vasoactive effects of bile salts in cirrhotic rats: in vivo and in vitro studies. Hepatology. 1993; 18(5): 1175-81.
- Das JB, Cosentino CM, Levy MF, Ansari GG, Raffensperger JG. Early hepatobiliary dysfunction during total parenteral nutrition: an experimental study. J Pediatr Surg. 1993; 28(1): 14-8.
- Fromm H, Malavolti M. Bile acid dissolution therapy of gallbladder stones. Baillieres Clin Gastroenterol. 1992; 6(4): 689-95.
- Tazuma S, Sasaki H, Mizuno S, Sagawa H, Hashiba S, Horiuchi I, Kajiyama G. Effect of ursodeoxycholic acid administration on nucleation time in human gallbladder bile. Gastroenterology. 1989; 97(1): 173-8.
|