|
Share this:
Read this article in PDF format
Pathogenetic treatment of functional gallbladder and sphincter of Oddi disorders
(E1, E2 and E3), functional dyspepsia (B1a and B1b) and symptomatic biliary
diseases with celecoxib and ursodeoxycholic acid (UDCA)
- Treatment of gallbladder dysfunction (with biliary pain) with/without biliary type III of sphincter of Oddi dysfunction and prophylaxis of chronic acalculous cholecystitis – celecoxib – 100 mg 2 times per day – 5-7 days, next UDCA – 750 mg 1 time before going to bed – 14 days.
Effectiveness of this treatment is 95% and a prolongation of remission period up to 18-24 months.
- Treatment of chronic acalculous cholecystitis (with biliary pain) with/without biliary type III of sphincter of Oddi dysfunction and prophylaxis of chronic acalculous cholecystitis with biliary sludge, atrophic antral gastritis (duodenogastric reflux and bile reflux gastritis), chronic biliary pancreatitis (biliopancreatic reflux) and chronic spastic aseptic pancreatitis (pancreatic type III of sphincter of Oddi dysfunction) – celecoxib – 100 mg 2 times per day – 5-7 days, next UDCA – 750 mg 1 time before going to bed – 30 days.
Effectiveness of this treatment is 95% and a prolongation of remission period up to 18-24 months.
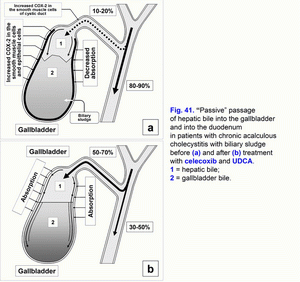
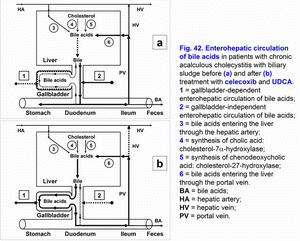
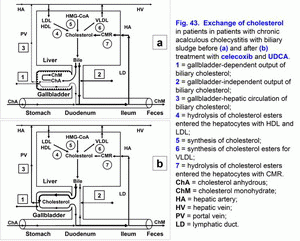
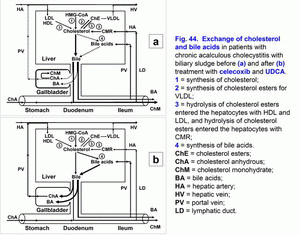
- Treatment of chronic acalculous cholecystitis with biliary sludge (with biliary pain) with/without biliary type III of sphincter of Oddi dysfunction and prophylaxis of chronic calculous cholecystitis, atrophic antral gastritis (duodenogastric reflux and bile reflux gastritis), chronic biliary pancreatitis (biliopancreatic reflux) and chronic spastic aseptic pancreatitis (pancreatic type III of sphincter of Oddi dysfunction) (fig. 41 a, b, c, d; fig. 41, 42, 43, 44) – celecoxib – 100 mg 2 times per day – 5-7 days, next UDCA – 750 mg 1 time before going to bed – 2 months.
Effectiveness of this treatment is 95% and a prolongation of remission period up to 19.3 ± 2.1 months.
- Treatment of chronic calculous cholecystitis (with biliary pain) with/without biliary type III of sphincter of Oddi dysfunction and prophylaxis of acute calculous cholecystitis, atrophic antral gastritis (duodenogastric reflux and bile reflux gastritis), chronic biliary pancreatitis (biliopancreatic reflux) and chronic spastic aseptic pancreatitis (pancreatic type III of sphincter of Oddi dysfunction) – celecoxib – 100 mg 2 times per day – 5-7 days, next UDCA – 750 mg 1 time before going to bed – 3 months.
Effectiveness of this treatment is 95% and a prolongation of remission period up to 18-24 months.
- Treatment of postcholecystectomy syndrome (with biliary pain) with/without biliary type III of sphincter of Oddi dysfunction and prophylaxis of choledocholithiasis, atrophic antral gastritis (duodenogastric reflux and bile reflux gastritis), chronic biliary pancreatitis (biliopancreatic reflux) and chronic spastic aseptic pancreatitis (pancreatic type III of sphincter of Oddi dysfunction) – celecoxib – 100 mg 2 times per day – 5-7 days, next UDCA – 750 mg 1 time before going to bed – 2 months.
Effectiveness of this treatment is 95% and a prolongation of remission period up to 18-24 months.
- Treatment of atrophic antral gastritis (duodenogastric reflux and bile reflux gastritis) (with biliary pain) and duodeno-gastroesophageal reflux (incompetence of Oddi's sphincter) – celecoxib – 100 mg 2 times per day – 5 days, next UDCA – 750 mg 1 time before going to bed – 14 days.
Estimated effectiveness of this treatment is 90-95% and a prolongation of remission period up to 18-24 months.
- Treatment of chronic biliary pancreatitis (biliopancreatic reflux) or chronic spastic aseptic pancreatitis (pancreatic type III of sphincter of Oddi dysfunction) (with biliary and/or pancreatic pain) – celecoxib – 100 mg 2 times per day – 7-10 days, next UDCA – 750 mg 1 time before going to bed – 30 days.
Estimated effectiveness of this treatment is 90-95% and a prolongation of remission period up to 18-24 months.
The pathogenetic correction of metabolic and morpho-functional disturbances in the gallbladder and liver in patients with gallbladder dysfunction helps decrease the risk of appearance of the chronic acalculous cholecystitis, in patients with chronic acalculous cholecystitis helps decrease the risk of appearance of the chronic calculous cholecystitis (fig. 41, fig. 42, fig. 43, fig. 44), in patients with chronic calculous cholecystitis helps decrease the risk of appearance of the acute calculous cholecystitis, in patients with postcholecystectomy syndrome helps decrease the risk of appearance of the choledocholithiasis.
Pathogenetic treatment with Celecoxib will help diminish the duration of disease period and the quantity of patients with biliary diseases by 30-40%.
Also, the remission period will be increased up to 18-24 months.
The conducted pathogenetic treatment of patients with chronic acalculous cholecystitis with biliary sludge promotes more effective controlling of biliary pain and inflammation in gallbladder wall, the restoration of excretion function of liver, the recovery of ejection fraction of gallbladder, and the restoration of the portal blood flow.
The pathogenetic treatment of patients with biliary diseases must include COX-2 inhibitors ( celecoxib) and UDCA.
Celecoxib is a selective inhibitor of COX-2. Inhibiting COX-2 activity in the smooth muscle cells of the gallbladder wall and cystic duct it brings relief of the biliary pain within 3-5 days, restoration of the evacuation function of the gallbladder and the gallbladder-dependent output of the biliary cholesterol, “active” and “passive” passage of the hepatic bile into the gallbladder, and decrease in the gallbladder-independent enterohepatic circulation of bile acids, biliary cholesterol and biliary bilirubin.
Celecoxib, a selective inhibitor of COX-2, inhibiting COX-2 activity in the epithelial cells of the gallbladder mucosa causes inhibition in glycoprotein mucin hypersecretion into the gallbladder lumen, decrease in concentration of glycoprotein biliary mucin in gallbladder bile and gallbladder bile viscosity, which prevents formation of biliary sludge.
Low COX-2 activity in the epithelial cells of the gallbladder mucosa helps in restoration of the absorption function of the gallbladder (absorption of water and biliary cholesterol), which results in increase in concentration of total bile acids and decrease in concentration of biliary cholesterol in gallbladder bile.
Ursodeoxycholic acid (UDCA) is a hydrophilic hepatoprotective bile acid. It helps in dissolving the cholesterol monohydrate crystals in the gallbladder, decrease in lithogenicity of gallbladder and hepatic bile, disappearance of the chronic “bland” intrahepatic cholestasis (i.e. results in restoration of the accumulation and excretion functions of liver).
Celecoxib and ursodeoxycholic acid (UDCA), blocking main pathogenetic mechanisms of gallstones formation, contribute to elimination of biliary sludge in 100% of cases, and lower the repeated risk of biliary sludge formation, and, respectively, reduce risk of gallstone formation in the gallbladder (1-66).
Celecoxib is a selective inhibitor of COX-2. Inhibiting COX-2 activity in the smooth muscle cells of the biliary tract and the sphincter of Oddi it brings relief of the biliary pain within 3-5 days, restoration of the passage of the hepatic bile into the duodenum.
Celecoxib is a selective inhibitor of COX-2, inhibiting COX-2 activity in the epithelial cells of the biliary tract mucosa causes decrease in secretion of glycoprotein mucin into the biliary tract lumen, concentration of the glycoprotein biliary mucin in the hepatic bile and viscosity of hepatic bile, which prevents formation of biliary sludge and gallstones in the common hepatic duct and common bile duct. Low COX-2 activity in the epithelial cells and the smooth muscle cells of the biliary tract helps in lowering the risk of choledocholithiasis development.
Ursodeoxycholic acid (UDCA) is a hydrophilic hepatoprotective bile acid. It helps in dissolving the cholesterol monohydrate crystals in the biliary tract, decrease in lithogenicity of hepatic bile, disappearance of the chronic “bland” intrahepatic cholestasis (i.e. results in the restoration of the accumulation and excretion functions of liver), and in some patients helps in dissolving the biliary sludge in the biliary tract.
Ursodeoxycholic acid (UDCA) is a hydrophilic hepatoprotective bile acid, decreasing aggressive properties of bile, prevents development of chronic atrophic antral gastritis (duodenogastric reflux and bile reflux gastritis) and duodeno-gastroesophageal reflux (incompetence of Oddi's sphincter), chronic biliary pancreatitis (biliopancreatic reflux) or chronic spastic aseptic pancreatitis (pancreatic type III of sphincter of Oddi dysfunction).
Celecoxib and Ursodeoxycholic acid (UDCA), pathogenetically blocking main mechanisms of gallstone formation, help in prophylactics of gallstone formation in the biliary tract, and lower the risk of development of choledocholithiasis and chronic biliary pancreatitis (1-66).
Attention!!! Information for patients:
Before using this scheme of treatment please check the contraindications (below) and side effects of using pharmacological preparations of Celecoxib and ursodeoxycholic acid, and obtain your doctor’s permission.
Contraindications for Celecoxib:
- allergic reactions (nettle-rash, bronchial spasm) to acetylsalicylic acid or other NSAIDs (in anamnesis);
- 3rd trimester of pregnancy;
- high sensitivity to sulphonamides;
- high sensitivity to any component of the preparation.
Contraindications for Ursodeoxycholic acid:
- high sensitivity to the preparation;
- acute inflammatory diseases of the gallbladder and the bile ducts;
- ulcerative colitis;
- Crone’s disease.
This web page does not bear any legal responsibility for the use of the proposed treatment schemes without consulting your doctor.
References (Celecoxib and UDCA):
- Chen XW, Cai JT. The impact of selective cycloxygenase-2 inhibitor celexibo on the formation of cholesterol gallstone. Zhonghua Nei Ke Za Zhi. 2003; 42(11): 797-9.
- Guarino MP, Carotti S, Sarzano M, Alloni R, Vanni M, Grosso M, Sironi G, Maffettone PL, Cicala M. Short-term ursodeoxycholic acid treatment improves gallbladder bile turnover in gallstone patients: a randomized trial. Neurogastroenterol Motil. 2005; 17(5): 680-6.
- Pazzi P, Petroni ML, Prandini N, Adam JA, Gullini S, Northfield TC, Jazrawi RP. Postprandial refilling and turnover: specific gallbladder motor function defects in patients with gallstone recurrence. Eur J Gastroenterol Hepatol. 2000; 12(7): 787-94.
- Ikegami T, Matsuzaki Y, Fukushima S, Shoda J, Olivier JL, Bouscarel B, Tanaka N. Suppressive effect of ursodeoxycholic acid on type IIA phospholipase A2 expression in HepG2 cells. Hepatology. 2005; 41(4): 896-905.
- Shoda J, Ueda T, Kawamoto T, Todoroki T, Asano T, Sugimoto Y, Ichikawa A, Maruyama T, Nimura Y, Tanaka N. Prostaglandin E receptors in bile ducts of hepatolithiasis patients and the pathobiological significance for cholangitis. Clin Gastroenterol Hepatol. 2003; 1(4): 285-96.
- Shoda J, Kano M, Asano T, Irimura T, Ueda T, Iwasaki R, Furukawa M, Kamiya J, Nimura Y, Todoroki T, Matsuzaki Y, Tanaka N. Secretory low-molecular-weight phospholipases A2 and their specific receptor in bile ducts of patients with intrahepatic calculi: factors of chronic proliferative cholangitis. Hepatology. 1999; 29(4): 1026-36.
- Tomida S, Abei M, Yamaguchi T, Matsuzaki Y, Shoda J, Tanaka N, Osuga T. Long-term ursodeoxycholic acid therapy is associated with reduced risk of biliary pain and acute cholecystitis in patients with gallbladder stones: a cohort analysis. Hepatology. 1999; 30(1): 6-13.
- Kano M, Shoda J, Irimura T, Ueda T, Iwasaki R, Urasaki T, Kawauchi Y, Asano T, Matsuzaki Y, Tanaka N. Effects of long-term ursodeoxycholate administration on expression levels of secretory low-molecular-weight phospholipases A2 and mucin genes in gallbladders and biliary composition in patients with multiple cholesterol stones. Hepatology. 1998; 28(2): 302-13.
- Shoda J, Ueda T, Ikegami T, Matsuzaki Y, Satoh S, Kano M, Matsuura K, Tanaka N. Increased biliary group II phospholipase A2 and altered gallbladder bile in patients with multiple cholesterol stones. Gastroenterology. 1997; 112(6): 2036-47.
- Carotti S, Guarino MP, Cicala M, Perrone G, Alloni R, Segreto F, Rabitti C, Morini S. Effect of ursodeoxycholic acid on inflammatory infiltrate in gallbladder muscle of cholesterol gallstone patients. Neurogastroenterol Motil. 2010.
- Guarino MP, Carotti S, Morini S, Perrone G, Behar J, Altomare A, Alloni R, Caviglia R, Emerenziani S, Rabitti C, Cicala M. Decreased number of activated macrophages in gallbladder muscle layer of cholesterol gallstone patients following ursodeoxycholic acid. Gut. 2008; 57(12): 1740-1.
- Jüngst C, Sreejayan N, Zündt B, Müller I, Spelsberg FW, Hüttl TP, Kullak-Ublick GA, del Pozo R, Jüngst D, von Ritter C. Ursodeoxycholic acid reduces lipid peroxidation and mucin secretagogue activity in gallbladder bile of patients with cholesterol gallstones. Eur J Clin Invest. 2008; 38(9): 634-9.
- Spier BJ, Pfau PR, Lorenze KR, Knechtle SJ, Said A. Risk factors and outcomes in post-liver transplantation bile duct stones and casts: A case-control study. Liver Transpl. 2008; 14(10): 1461-5.
- Guarino MP, Cong P, Cicala M, Alloni R, Carotti S, Behar J. Ursodeoxycholic acid improves muscle contractility and inflammation in symptomatic gallbladders with cholesterol gallstones. Gut. 2007; 56(6): 815-20.
- Mas MR, Comert B, Mas N, Yamanel L, Ozotuk H, Tasci I, Jazrawi RP. Effects of long term hydrophilic bile acid therapy on in vitro contraction of gallbladder muscle strips in patients with cholesterol gallstones. World J Gastroenterol. 2007; 13(32): 4336-9.
- Jüngst C, Sreejayan N, Eder MI, von Stillfried N, Zündt B, Spelsberg FW, Kullak-Ublick GA, Jüngst D, von Ritter C. Lipid peroxidation and mucin secretagogue activity in bile of gallstone patients. Eur J Clin Invest. 2007; 37(9): 731-6.
- Itoh S, Kono M, Akimoto T. Psoriasis treated with ursodeoxycholic acid: three case reports. Clin Exp Dermatol. 2007; 32(4): 398-400.
- Beuers U. Drug insight: Mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2006; 3(6): 318-28.
- Colecchia A, Mazzella G, Sandri L, Azzaroli F, Magliuolo M, Simoni P, Bacchi-Reggiani ML, Roda E, Festi D. Ursodeoxycholic acid improves gastrointestinal motility defects in gallstone patients. World J Gastroenterol. 2006; 12(33): 5336-43.
- Pemberton PW, Aboutwerat A, Smith A, Warnes TW. Ursodeoxycholic acid in primary biliary cirrhosis improves glutathione status but fails to reduce lipid peroxidation. Redox Rep. 2006; 11(3): 117-23.
- Jeong HJ, Kim CG. Pretreatment with ursodeoxycholic acid (UDCA) as a novel pharmacological intervention in hepatobiliary scintigraphy. Yonsei Med J. 2005; 46(3): 394-8.
- Fischer S, Müller I, Zündt BZ, Jüngst C, Meyer G, Jüngst D. Ursodeoxycholic acid decreases viscosity and sedimentable fractions of gallbladder bile in patients with cholesterol gallstones. Eur J Gastroenterol Hepatol. 2004; 16(3): 305-11.
- Sauter GH, Thiessen K, Parhofer KG, Jüngst C, Fischer S, Jüngst D. Effects of ursodeoxycholic acid on synthesis of cholesterol and bile acids in healthy subjects. Digestion. 2004; 70(2): 79-83.
- Xiao ZL, Biancani P, Carey MC, Behar J. Hydrophilic but not hydrophobic bile acids prevent gallbladder muscle dysfunction in acute cholecystitis. Hepatology. 2003; 37(6): 1442-50.
- Tazuma S, Nishioka T, Ochi H, Hyogo H, Sunami Y, Nakai K, Tsuboi K, Asamoto Y, Sakomoto M, Numata Y, Kanno K, Yamaguchi A, Kobuke T, Komichi D, Nonaka Y, Chayama K. Impaired gallbladder mucosal function in aged gallstone patients suppresses gallstone recurrence after successful extracorporeal shockwave lithotripsy. J Gastroenterol Hepatol. 2003; 18(2): 157-61.
- Gunsar C, Melek M, Karaca I, Sencan A, Mir E, Ortac R, Canan O. The biochemical and histopathological effects of ursodeoxycholic acid and metronidazole on total parenteral nutrition-associated hepatic dysfunction: an experimental study. Hepatogastroenterology. 2002; 49(44): 497-500.
- Xiao ZL, Rho AK, Biancani P, Behar J. Effects of bile acids on the muscle functions of guinea pig gallbladder. Am J Physiol Gastrointest Liver Physiol. 2002; 283(1): G87-94.
- Kano M, Shoda J, Satoh S, Kobayashi M, Matsuzaki Y, Abei M, Tanaka N. Increased expression of gallbladder cholecystokinin: a receptor in prairie dogs fed a high-cholesterol diet and its dissociation with decreased contractility in response to cholecystokinin. J Lab Clin Med. 2002; 139(5): 285-94.
- Wang DQ, Tazuma S. Effect of beta-muricholic acid on the prevention and dissolution of cholesterol gallstones in C57L/J mice. J Lipid Res. 2002; 43(11): 1960-8.
- Lukivskaya OY, Maskevich AA, Buko VU. Effect of ursodeoxycholic acid on prostaglandin metabolism and microsomal membranes in alcoholic fatty liver. Alcohol. 2001; 25(2): 99-105.
- Bomzon A, Ljubuncic P. Ursodeoxycholic acid and in vitro vasoactivity of hydrophobic bile acids. Dig Dis Sci. 2001; 46(9): 2017-24.
- Sunami Y, Tazuma S, Kajiyama G. Gallbladder dysfunction enhances physical density but not biochemical metastability of biliary vesicles. Dig Dis Sci. 2000; 45(12): 2382-91.
- Ljubuncic P, Said O, Ehrlich Y, Meddings JB, Shaffer EA, Bomzon A. On the in vitro vasoactivity of bile acids. Br J Pharmacol. 2000; 131(3): 387-98.
- Nishioka T, Tazuma S, Yamashita G, Kajiyama G. Partial replacement of bile salts causes marked changes of cholesterol crystallization in supersaturated model bile systems. Biochem J. 1999; 340 ( Pt 2): 445-51.
- Sinisalo J, Vanhanen H, Pajunen P, Vapaatalo H, Nieminen MS. Ursodeoxycholic acid and endothelial-dependent, nitric oxide-independent vasodilatation of forearm resistance arteries in patients with coronary heart disease. Br J Clin Pharmacol. 1999; 47(6): 661-5.
- van de Heijning BJ, van de Meeberg PC, Portincasa P, Doornewaard H, Hoebers FJ, van Erpecum KJ, Vanberge-Henegouwen GP. Effects of ursodeoxycholic acid therapy on in vitro gallbladder contractility in patients with cholesterol gallstones. Dig Dis Sci. 1999; 44(1): 190-6.
- Mendez-Sanchez N, Brink MA, Paigen B, Carey MC. Ursodeoxycholic acid and cholesterol induce enterohepatic cycling of bilirubin in rodents. Gastroenterology. 1998; 115(3): 722-32.
- Benedetti A, Alvaro D, Bassotti C, Gigliozzi A, Ferretti G, La Rosa T, Di Sario A, Baiocchi L, Jezequel AM. Cytotoxicity of bile salts against biliary epithelium: a study in isolated bile ductule fragments and isolated perfused rat liver. Hepatology. 1997; 26(1): 9-21.
- Ohtake M, Sandoh N, Sakaguchi T, Tsukada K, Hatakeyama K. Enhancement of portal blood flow by ursodesoxycholic acid in partially hepatectomized rats. Surg Today. 1996; 26(2): 142-4.
- Fahey DA, Carey MC, Donovan JM. Bile acid/phosphatidylcholine interactions in mixed monomolecular layers: differences in condensation effects but not interfacial orientation between hydrophobic and hydrophilic bile acid species. Biochemistry. 1995; 34(34): 10886-97.
- Bouscarel B, Ceryak S, Robins SJ, Fromm H. Studies on the mechanism of the ursodeoxycholic acid-induced increase in hepatic low-density lipoprotein binding. Lipids. 1995; 30(7): 607-17.
- Bomzon A, Ljubuncic P. Bile acids as endogenous vasodilators? Biochem Pharmacol. 1995; 49(5): 581-9.
- Jazrawi RP, Pazzi P, Petroni ML, Prandini N, Paul C, Adam JA, Gullini S, Northfield TC. Postprandial gallbladder motor function: refilling and turnover of bile in health and in cholelithiasis. Gastroenterology. 1995; 109(2): 582-91.
- Pak JM, Adeagbo AS, Triggle CR, Shaffer EA, Lee SS. Mechanism of bile salt vasoactivity: dependence on calcium channels in vascular smooth muscle. Br J Pharmacol. 1994; 112(4): 1209-15.
- Sasaki H, Tazuma S, Kajiyama G. Effects of 16,16-dimethyl prostaglandin E2 on biliary mucous glycoprotein and gallstone formation in guinea pigs. Scand J Gastroenterol. 1993; 28(6): 495-9.
- Mizuno S, Tazuma S, Kajiyama G. Stabilization of biliary lipid particles by ursodeoxycholic acid. Prolonged nucleation time in human gallbladder bile. Dig Dis Sci. 1993; 38(4): 684-93.
- Pak JM, Lee SS. Vasoactive effects of bile salts in cirrhotic rats: in vivo and in vitro studies. Hepatology. 1993; 18(5): 1175-81.
- Das JB, Cosentino CM, Levy MF, Ansari GG, Raffensperger JG. Early hepatobiliary dysfunction during total parenteral nutrition: an experimental study. J Pediatr Surg. 1993; 28(1): 14-8.
- Fromm H, Malavolti M. Bile acid dissolution therapy of gallbladder stones. Baillieres Clin Gastroenterol. 1992; 6(4): 689-95.
- Tazuma S, Sasaki H, Mizuno S, Sagawa H, Hashiba S, Horiuchi I, Kajiyama G. Effect of ursodeoxycholic acid administration on nucleation time in human gallbladder bile. Gastroenterology. 1989; 97(1): 173-8.
|